I was asked recently why male mice are not used for breast cancer work – here is my long answer.
Experimental biology relies to a large extent on models that can be manipulated in the laboratory. In general, the simpler the model, the easier it is to understand and use, but the further it is removed from the disease. Let’s call these extremes “easy” and “faithful“.
In relation to cancer, you can learn some things from very easy models like isolated cells grown in a dish, or a fruit fly. More faithful to the human disease are animal models, and these become particularly important when you want to do work that leads to clinical studies in humans. Mouse models are common because mice are convenient lab animals and manipulating the mouse genome is well understood.
There are two broad categories of cancer mouse models: models where the cancer is implanted by the scientist and models where the animal is treated with a carcinogen or has its genome manipulated so that cancers arise “spontaneously”. Many models of both kinds exist for breast cancer, but none of them model the male disease, to my knowledge (if you know different, please reach out!).
There are many considerations related to animal models, including ethical considerations, which I will not get into here. Please see the wikipedia article here.
Implant Models – easier
As the name implies, an implant model entails a scientist taking cancer cells from a dish or another mouse, and implanting them into a naive mouse to observe the cancer’s growth for any number of studies. This is relatively easy to do.
Implants have the drawback that you are introducing something foreign into an animal. If you are using human tumors in mice, you have to work with mice whose immune system doesn’t reject the foreign tissue, which would preclude, for example, many immunotherapy related studies. These kinds of considerations mean that implant models are considered less faithful than other mouse models.
There are sub-categories of implant models, again broadly speaking:
- Ectopic means you put the cancer somewhere other than where it should be. Often this is done under the skin, because it is very easy to follow a tumors growth when it is a measurable bump. Of course, that is not where most tumors grow.
- Orthotopic means putting the tumor where it is ordinarily found. A glioma in the brain, for example.
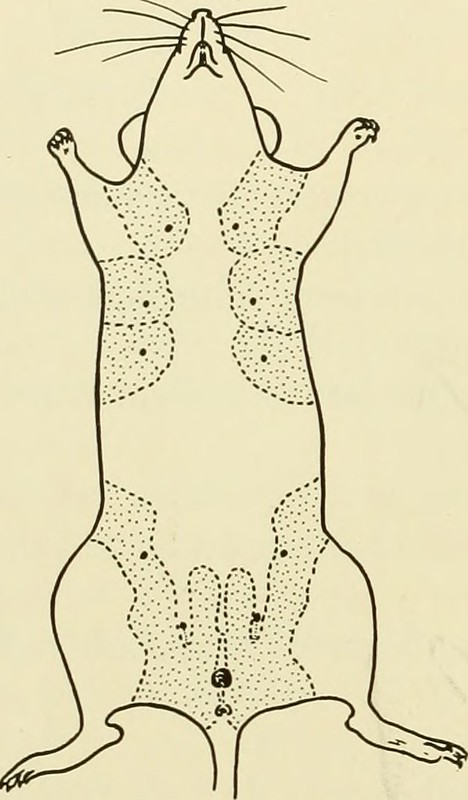
When you are working with a female breast cancer the orthotopic site is the mammary fat pad, the breast tissue of the mouse (see picture below from a 1940’s book published by Jax). This is hard in female mice, and harder in male mice which also don’t have the ductal structures that are considered the tissue of origin for most male breast cancers.
(From: https://archive.org/stream/biologyoflaborat00rosc/#page/174/mode/1up via Flickr.)
Of course you have to also consider that the male breast is only the orthotopic site for a male breast cancer cell, and putting a female breast cancer into a male breast is likely not totally orthotopic. Differences observed between male and female breast cancers are probably at least in part due to where they grow.
So, what is currently possible is the ectopic implantation of female breast cancers into male mice – either under the skin (easy) or into the vestigial male breast (hard). Neither of these are very faithful and so not very informative. For this reason such experiments are not likely to meet with approval by ethical regulators or peer reviewers for journals or funding agencies.
Genetic Models
Genetic models are on the whole more faithful. Cancers arise from the tissue of the model animal and follow a path that is similar to the human disease, although this is usually accelerated and simplified. Such models are made by altering genes that are in critical pathways for the particular cancer, which is based on studies of the human disease. Many, many models exist and many have been made for breast cancer.
Genetic are also less easy. Even in the best cancer models, not every mouse may get the cancer. It may take weeks or months for the cancer to appear. Often the rarer the cancer in humans, the more this is the case. In the implant models above you can take 10 mice and a skilled experimenter can generate 10 cancers, one in each, pretty much every time. They will know that it takes, say, 2 weeks for the tumors to reach a certain size. This makes it easy to plan and execute an experiment. In the genetic equivalent, the experimenter may need to breed 100 mice, and surveil them constantly to eventually find the 10 that develop the tumor, which may occur anywhere between 6 and 12 weeks. Mileage varies a lot – some models have near 100% penetrance and tumors arise quickly (easier end of the genetic spectrum) but others can take months or years and be relatively rare (faithful end).
It costs money to house mice. If you are working on a genetic model of breast cancer most investigators cull male mice in their litters. They keep some male mice so that they can breed future generations of the model, and I know anecdotally that sometimes these male mice also develop breast cancers. This is not surprising, as male and female breast cancers have many common genetic pathways (like BRCA). But it is very rare. If you have a model where half the female mice get breast cancer, it may be one in a 100 for males. So to do a reasonable experiment on male mice, you’d need to breed and screen many thousands of mice. Costs and ethics therefore mean it is not feasible to use a female breast cancer model to study the male disease.
What we need is a male model
No. Not that kind of male model. A mouse model that is genetically focused on the male breast.
This would be a model that make cancer-relevant changes that are specifically targeted at the male breast or have a very high penetrance on the male side. Almost 20 years ago a mouse model for gynecomastia in males was published, showing some of the principles that could be applied.
In the past decades genetic technology has advanced rapidly and you can now envisage models that combine germline genetics which predispose to breast cancer being combined with topical manipulations that focus and accelerate the carcinogenic process. You could take a female model for breast cancer, e.g. with an alteration in the BRCA pathway, and add focused gene editing in the mouse male breast tissue might allow you to radically bump up the yield of tumors in a way that begins to make it possible to do meaningful studies. Unfortunately, such models are not easy, and arguably less faithful than less manipulated models, but it is a compromise that might make sense. Until we have such models it will be difficult to test hypotheses directly.

Very good to know about breast cancer and it improves my knowledge as well. Very well written with genetic model of mammal. Thanks for sharing information.